With a total annual healthcare spending of $1.94 trillion, access to the EU healthcare market is crucial to success in the medical device industry. But in order to enter this critical market, companies need to obtain a CE Mark certifying that their medical device has passed EU requirements. Moreover, all documentation must be translated into the official language(s) of the EU member countries, and non-EU countries following the same procedure – meaning medical device companies must take into account CE mark translation requirements as well.
What is CE Marking?

Many people commonly refer to CE Marking as a quality mark when in reality it is a safety mark, meaning that the product has been assessed with regard to safety, health, and environmental protection requirements to be sold in the European Union. In order for the product to pass the safety assessment, it must meet certain CE marking requirements – including medical device translation for labels and product information.
CE Mark Translation Requirements
Medical device translation requirements state that companies must provide the respective authority in each country they wish to sell their product with clinically-relevant safety and efficacy data for each of their treatment’s indications. This must be done in order to market medical devices in the EU under the new regulations. This mandatory data is typically found in three documents: Instructions for Use (IFU), Labeling information, and Packaging information. However, companies may also be required to translate the User Interface (UI) and software package, marketing materials or patient/caregiver details, depending on the complexity and nature of the device.
CE mark translation requirements state that translations in the official languages of each country are mandatory process. The languages required are generally the country’s official languages or those spoken by a majority of the population. Nevertheless, when applying for a CE Mark, translating the required materials into each of the “Big 5” languages should be a priority: German, Italian, Spanish, French, and English, since translations for regulatory authorities typically involve at least one of these.
CE Mark Translation Requirements Will Depend On The Country
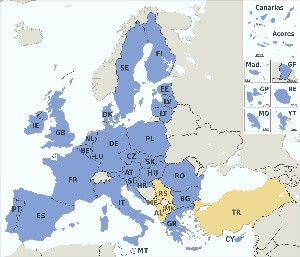
The CE marking, and as such CE marking translation requirements, are usually required in the following countries:
| 1. Austria | 9. France | 19. Luxembourg | 26. Slovakia |
| 2. Belgium | 10. Germany | 20. Malta | 27. Slovenia |
| 3. Bulgaria | 11. Greece | 21. Netherlands | 28. Spain |
| 4. Czech Republic | 12. Hungary | 22. Norway | 29. Sweden |
| 5. Cyprus | 13. Iceland | 23. Poland | 30. United Kingdom |
| 6. Denmark | 14. Ireland | 24. Portugal | 31. Croatia (2013) |
| 7. Estonia | 15. Italy | 25. Romania | |
| 8. Finland | 16. Latvia | 19. Luxembourg |
Switzerland is not a member of the European Union, but for some products it accepts the CE marking as a presumption of conformity with Swiss national technical regulations.
Turkey and the CEFTA countries (Macedonia, Albania, Bosnia and Herzegovina, Moldova, Montenegro, Serbia and Kosovo) are neither members of the EU, nor are considered a part of the European Economic Area (EEA). However, they are already adopting many of the European CE marking directives.
So when applying for a CE mark, the translation into one of the “Big 5” languages may not be enough. It is important to contact the appointed Competent Authority in each country for guidance, since the translation into the official language of any of the countries listed above may be required.
High-quality medical translations are the insurance for achieving future regulatory approval and low-risk adoption of any treatment among end users. Low quality translation services put product, patients and reputation at risk. Make sure to mitigate the risk by partnering with an ISO-complying Language Service Provider (LSP), experienced in life sciences translation and medical device translation, to translate medical documents required for obtaining a CE Mark.
About Language Connections:
Language Connections is one of the top language service companies in the US. Over the last 30 years, we’ve focused on providing the best business translation services, interpreting services, as well as interpreter training and customized language training programs. In addition to top-tier corporate language training, we offer certified corporate interpreters and professional business translation services in 200+ languages. Our network includes linguists with backgrounds in all major industries. They’re ready to meet your needs, whether they’re for technical translation services, legal translation, government translation services, international development translation services, education translation services, life sciences translation, or something else. Reach out to us today for a free quote on our cost-efficient and timely translation services, interpreters, or other linguistic services.
Language Connections Inc.
2001 Beacon Street, Suite 105,
Boston, MA 02135
Phone: +1-617-731-3510
Email: service@languageconnections.com


